Catharanthus Roseus Extract Powder
Catharanthus roseus extract powder is a powdered form of an extract derived from the Catharanthus roseus plant, also known as the Madagascar periwinkle or the rosy periwinkle. This plant is known for its medicinal properties and contains various bioactive compounds, including alkaloids such as vinblastine and vincristine, which have been studied for their potential anti-cancer properties.
The extract powder is typically obtained through the extraction of bioactive compounds from the plant material and then processed into a powdered form for various applications. It may be used in traditional medicine, pharmaceuticals, or research settings due to its potential medicinal properties.
Catharanthus roseus is known for being a legendary medicinal plant because it contains two antitumor terpenoid indole alkaloids (TIAs), vinblastine and vincristine. In traditional Chinese medicine, extracts from the plant have been used to treat diseases such as malaria, diabetes, and Hodgkin's lymphoma. In the 1950s, vinca alkaloids were isolated from Catharanthus roseus while screening for anti-diabetic drugs.
Catharanthus roseus, commonly known as bright eyes, Cape periwinkle, graveyard plant, Madagascar periwinkle, old maid, pink periwinkle, or rose periwinkle, is a perennial species of flowering plant in the family Apocynaceae. It is native and endemic to Madagascar but is grown elsewhere as an ornamental and medicinal plant, and now has a pantropical distribution. It is a source of the drugs vincristine and vinblastine, used to treat cancer. It was formerly included in the genus Vinca as Vinca rosea. It has many vernacular names among which are arivotaombelona or rivotambelona, tonga, tongatse or trongatse, tsimatiririnina, and vonenina.
| Main Active Ingredients in Chinese | English Name | CAS No. | Molecular Weight | Molecular Formula |
| 长春胺 | Vincamine | 1617-90-9 | 354.44 | C21H26N2O3 |
| 脱水长春碱 | Anhydrovinblastine | 38390-45-3 | 792.96 | C46H56N4O8 |
| 異長春花苷內酰胺 | Strictosamide | 23141-25-5 | 498.53 | C26H30N2O8 |
| 四氢鸭脚木碱 | Tetrahydroalstonine | 6474-90-4 | 352.43 | C21H24N2O3 |
| 酒石酸长春瑞滨 | Vinorelbine Tartrate | 125317-39-7 | 1079.12 | C45H54N4O8.2(C4H6O6);C |
| 长春瑞滨 | Vinorelbine | 71486-22-1 | 778.93 | C45H54N4O8 |
| 长春新碱 | Vincristine | 57-22-7 | 824.96 | C46H56N4O10 |
| 硫酸长春新碱 | Vincristine sulfate | 2068-78-2 | 923.04 | C46H58N4O14S |
| 硫酸长春质碱 | Catharanthine Sulfate | 70674-90-7 | 434.51 | C21H26N2O6S |
| 酒石酸长春质碱 | Catharanthine hemitartrate | 4168-17-6 | 486.51 | C21H24N2O2.C4H6O6 |
| 长春花碱 | Vinblastine | 865-21-4 | 810.99 | C46H58N4O9 |
| 长春质碱 | Catharanthine | 2468-21-5 | 336.43 | C21H24N2O2 |
| 文朵灵 | Vindoline | 2182-14-1 | 456.53 | C25H32N2O6 |
| 硫酸长春碱 | Vinblastine Sulfate | 143-67-9 | 909.05 | C46H60N4O13S |
| β-谷甾醇 | β-Sitosterol | 83-46-5 | 414.71 | C29H50O |
| 菜油甾醇 | Campesterol | 474-62-4 | 400.68 | C28H48O |
| 齐墩果酸 | Oleanolic acid | 508-02-1 | 456.7 | C30H48O3 |
| PRODUCT SPECIFICATIONS | ||
| Product Name: | Vinca rosea extact | |
| Botanic Name: | Catharanthus roseus (L.) | |
| Part of plant | Flower | |
| Country of Origin: | China | |
| ANALYSIS ITEMS | SPECIFICATION | TEST METHOD |
| Appearance | Fine powder | Organoleptic |
| Color | Brown fine powder | Visual |
| Odour & Taste | Characteristic | Organoleptic |
| Identification | Identical to R.S. sample | HPTLC |
| Extract Ratio | 4:1~20:1 | |
| Sieve Analysis | 100% through 80 mesh | USP39 <786> |
| Loss on drying | ≤ 5.0% | Eur.Ph.9.0 [2.5.12] |
| Total Ash | ≤ 5.0% | Eur.Ph.9.0 [2.4.16] |
| Lead (Pb) | ≤ 3.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Arsenic (As) | ≤ 1.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Cadmium(Cd) | ≤ 1.0 mg/kg | Eur.Ph.9.0<2.2.58>ICP-MS |
| Mercury(Hg) | ≤ 0.1 mg/kg -Reg.EC629/2008 | Eur.Ph.9.0<2.2.58>ICP-MS |
| Heavy metal | ≤ 10.0 mg/kg | Eur.Ph.9.0<2.4.8> |
| Solvents Residue | Conform Eur.ph. 9.0 <5,4 > and EC European Directive 2009/32 | Eur.Ph.9.0<2.4.24> |
| Pesticides Residues | Conform Regulations(EC) No.396/2005
including annexes and successive updates Reg.2008/839/CE |
Gas Chromatography |
| Aerobic bacteria(TAMC) | ≤10000 cfu/g | USP39 <61> |
| Yeast/Moulds(TAMC) | ≤1000 cfu/g | USP39 <61> |
| Escherichia coli: | Absent in 1g | USP39 <62> |
| Salmonella spp: | Absent in 25g | USP39 <62> |
| Staphylococcus aureus: | Absent in 1g | |
| Listeria Monocytogenens | Absent in 25g | |
| Aflatoxins B1 | ≤ 5 ppb -Reg.EC 1881/2006 | USP39 <62> |
| Aflatoxins ∑ B1, B2, G1, G2 | ≤ 10 ppb -Reg.EC 1881/2006 | USP39 <62> |
Catharanthus roseus Extract Powder, or Vinca rosea extract, derived from the Madagascar periwinkle plant, possesses several notable characteristics:
Bioactive Compounds: The extract powder contains bioactive compounds such as vinblastine and vincristine, which are known for their potential medicinal properties, particularly in the field of cancer treatment.
Medicinal Properties: The extract powder is valued for its potential medicinal benefits, including anti-cancer, anti-diabetic, and anti-hypertensive properties, among others.
Natural Sourcing: It is sourced from the Catharanthus roseus plant, known for its natural occurrence and traditional medicinal uses.
Pharmaceutical Applications: The extract powder is suitable for use in pharmaceutical formulations and research due to its bioactive nature and potential therapeutic applications.
Quality and Purity: The product is manufactured to high-quality standards, ensuring purity, potency, and consistency in its bioactive compound content.
Research Interest: It is of interest to researchers and healthcare professionals due to its potential in developing new pharmaceutical products and treatments.
Here are the health benefits of Catharanthus roseus Extract Powder in short sentences:
1. Potential anti-cancer properties attributed to the presence of vinblastine and vincristine alkaloids.
2. Research suggests anti-diabetic effects, potentially aiding in blood sugar management.
3. Possible use in hypertension management due to its reported hypotensive properties.
4. Investigated for its antimicrobial and antiviral potential in supporting immune health.
5. Research interest in its neuroprotective properties for cognitive health support.
6. Potential application in skincare formulations due to its reported antioxidant properties.
7. Studied for its anti-inflammatory effects, which may have implications for various health conditions.
8. Investigated for its potential to support overall wellness and vitality.
1. Anti-cancer formulations and research due to the presence of vinblastine and vincristine alkaloids.
2. Development of anti-diabetic medications and supplements.
3. Potential use in hypertension management and related pharmaceuticals.
4. Research into novel therapeutic agents for various medical conditions.
5. Ingredient in traditional medicine and herbal remedies.
6. Exploration of its properties for skincare and cosmetic formulations.
7. Investigation for its potential in treating microbial infections.
8. Development of dietary supplements for overall health and wellness support.
9. Research into its neuroprotective and cognitive health benefits.
10. Potential applications in veterinary medicine and animal health products.
These applications highlight the diverse potential uses of Catharanthus roseus Extract Powder across pharmaceuticals, healthcare, wellness, and research sectors.
Catharanthus roseus Extract Powder, like many natural products, may have potential side effects, especially when used in concentrated forms. Some potential side effects may include:
Gastrointestinal Disturbances: Such as nausea, vomiting, or diarrhea in some individuals.
Hypotension: Due to its reported hypotensive properties, excessive use may lead to low blood pressure.
Neurological Effects: High doses may lead to neurological symptoms such as dizziness or confusion.
Allergic Reactions: Some individuals may experience allergic reactions, especially if they have known plant allergies.
Drug Interactions: It may interact with certain medications, so caution is advised, especially for individuals on other medications.
It’s important to consult with a healthcare professional before using Catharanthus roseus Extract Powder, especially if you have underlying health conditions or are taking medications. This will help ensure its safe and appropriate use.
Packaging And Service
Packaging
* Delivery Time: Around 3-5 workdays after your payment.
* Package: In fiber drums with two plastic bags inside.
* Net Weight: 25kgs/drum,Gross Weight: 28kgs/Drum
* Drum Size & Volume: I.D.42cm × H52cm, 0.08 m³/ Drum
* Storage: Stored in a dry and cool place, keep away from strong light and heat.
* Shelf Life: Two years when properly stored.
Shipping
* DHL Express, FEDEX, and EMS for quantities less than 50KG, usually called as DDU service.
* Sea shipping for quantities over 500 kg; and air shipping is available for 50 kg above.
* For high-value products, please select air shipping and DHL express for safety.
* Please confirm if you can make the clearance when goods reach your customs before placing an order. For buyers from Mexico, Turkey, Italy, Romania, Russia, and other remote areas.
Payment And Delivery Methods
Express
Under 100kg, 3-5 Days
Door to door service easy to pick up the goods
By Sea
Over300kg, Around 30 Days
Port to port service professional clearance broker needed
By Air
100kg-1000kg, 5-7 Days
Airport to airport service professional clearance broker needed
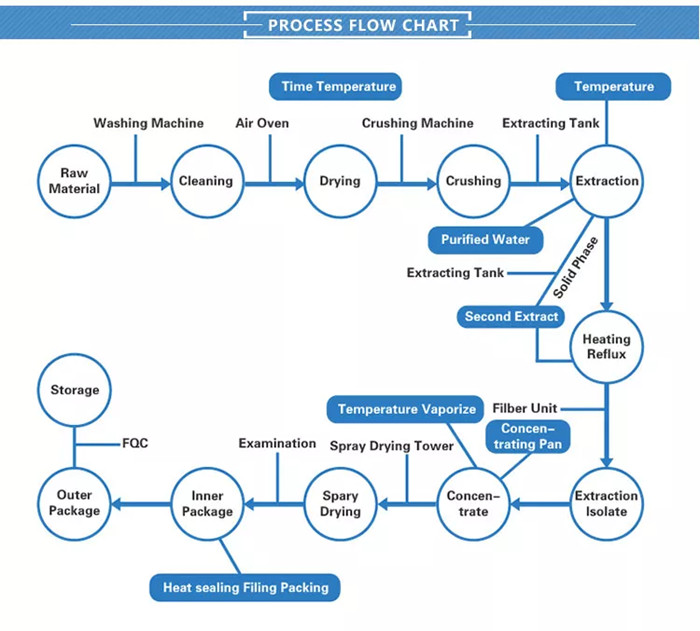
Production Details (Flow Chart)
1. Sourcing and Harvesting
2. Extraction
3. Concentration and Purification
4. Drying
5. Standardization
6. Quality Control
7. Packaging 8. Distribution
Certification
It is certified by ISO, HALAL, and KOSHER certificates.